Thermodynamics is basically a branch of science and deals with heat, work, temperature and energy. In other words, thermodynamics deals with the transfer of energy from one place to another and from one form to another.
Heat transfer between objects or systems by conduction, convection and radiation is also within the scope of Thermodynamics.
Sub-branches of Thermodynamics
Thermodynamics is divided into several branches as follows.
- Classical Thermodynamics
- Statistical Thermodynamics
- Chemical Thermodynamics
- Equilibrium Thermodynamics
- Non-Equilibrium Thermodynamics
Let’s briefly explain the sub-branches of thermodynamics.
Classical Thermodynamics
In classical thermodynamics, matter and its behavior are studied at the macroscopic level. Temperature and pressure are taken into account when determining the properties of the system or substance.
Statistical Thermodynamics
In statistical thermodynamics, the interpretation of microscopic interactions between particles or quantum mechanical states was added to classical thermodynamics. This field was explained as a natural consequence of classical thermodynamics, statistics, classical mechanics and quantum theory at the microscopic level.
Chemical Thermodynamics
Chemical thermodynamics is the study of how energy interacts with chemical processes or changes of state in accordance with the laws of thermodynamics.
Equilibrium Thermodynamics
Equilibrium thermodynamics is the study of matter and energy transfers in systems or substances that can be moved from one state of thermodynamic equilibrium to another by agents in their environment. The phrase “thermodynamic equilibrium” refers to a state of equilibrium in which all macroscopic fluxes are zero.
Non-equilibrium Thermodynamics
Systems that are not in thermodynamic equilibrium are the focus of this field of thermodynamics. The majority of systems in nature are not in thermodynamic equilibrium because they are not in a steady state and are subject to constant and irregular flows of matter and energy from other systems.
Laws of Thermodynamics
The laws of thermodynamics are the four laws that form the basis of thermodynamics. It defines the structure of heat and work transfers in thermodynamic processes.
The laws of thermodynamics have very general validity and do not vary depending on the details of the interactions or the properties of the system under consideration. In other words, even if only the matter or energy input and output of a system is known, it can be applied to this system.
Laws of Thermodynamics:
- Zeroth Law of Thermodynamics
- First Law of Thermodynamics
- Second Law of Thermodynamics
- Third Law of Thermodynamics
Zeroth Law of Thermodynamics
Although two systems interact with each other, if their states remain unchanged, these two systems are said to be in balance with each other. If two systems are open to interaction, but there is no net energy transfer (heat transfer) between them other than the energy transfer (work) through mechanical interaction, these two systems are in thermal balance with each other. The zeroth law says:
If systems A and B are in thermal equilibrium with each other, a system C that is in thermal equilibrium with system A is also in thermal equilibrium with system B.
This state of equilibrium is defined as temperature. That is, each degree of temperature represents a different state of equilibrium. This situation:
It is formulated as TA=TB=TC
First Law of Thermodynamics
The first law of thermodynamics is also known as “conservation of energy”. According to this law:
The amount of change in the internal energy of a system is equal to the difference between the amount of heat added to that system and the work applied by the system to its environment.
This situation is shown as follows:
U2 – U1 = Q – W
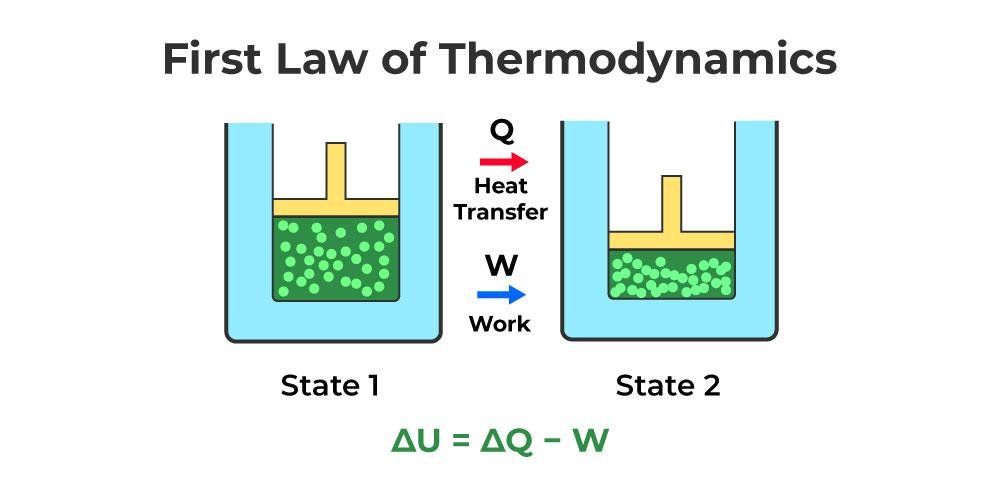
Energy cannot be created or destroyed, it can only be transformed from one form to another. For any cycle of a system, heat exchange and work exchange during the cycle must be equal to each other in the same unit system and proportional to each other in different unit systems. The accuracy of these statements has been observed through experiments, but they cannot be proven. All these expressions can be expressed much more easily mathematically.
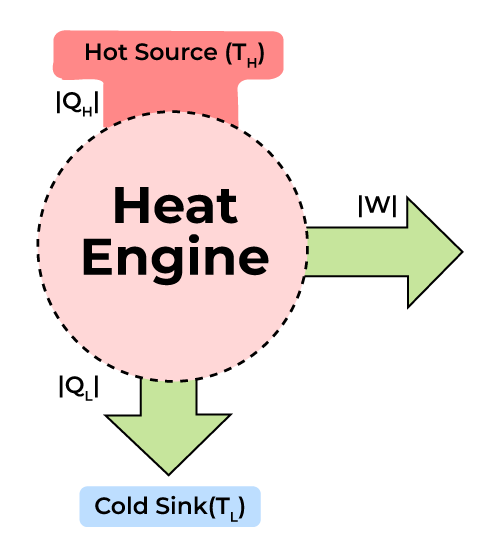
Second Law of Thermodynamics
The second law of thermodynamics is as follows:
Heat cannot flow spontaneously from a cold area to a hot area; In other words, heat at a certain temperature cannot be completely converted into work.
As a result, the entropy of a closed system reaches its highest value over time. That is, all closed systems tend to reach a state of equilibrium, and in this state of equilibrium, when entropy is at its highest level, the amount of energy that can do work is zero. This back-and-forth asymmetric process has led to the creation of the concept called “time arrow” in physics.
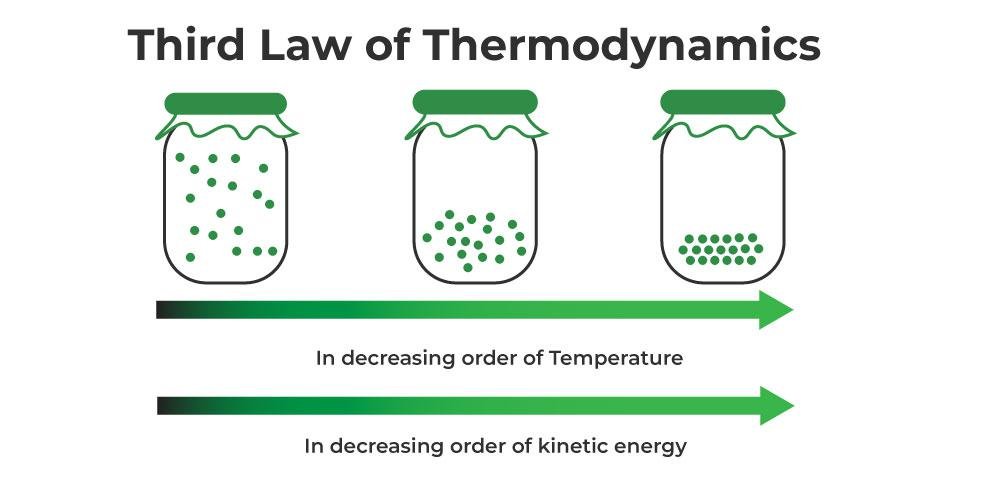
Third Law of Thermodynamics
This law states why it is impossible to cool a substance to absolute zero:
As the temperature approaches absolute zero, all movements approach zero.
As the temperature approaches absolute zero, the entropy of a system approaches a constant. The reason why this number is a constant rather than zero is that, although all movements have stopped and the associated uncertainties have disappeared, there is still an uncertainty arising from the different molecular arrangements of non-crystalline substances. In addition, thanks to the third law, absolute entropy, which is very useful in examining chemical reactions, can be defined by taking the entropy of substances at absolute zero as a reference.