Heat transfer is used in all areas of life to transfer thermal energy from one place to another. For example, the Sun’s heating of the Earth is possible through heat transfer. Although the branch of thermodynamics deals with heat transfer, many engineering uses it.
Heat transfer basically refers to the flow of heat from where it is high to where it is low. Heat transfer continues until two objects or two systems with heat transfer between them reach thermal equilibrium.
Heat Transfer Formula
The heat transfer formula determines the amount of heat transferred from one system to another. The heat transfer formula is the basic formula and other formulas are derived from this equation.
Q = c × m × ΔT
Q: is the heat supplied to the system
m: is the mass of the system
c: is the specific heat capacity of the system
ΔT: is the change in temperature of the system
The specific heat capacity (c) is defined as the quantity of heat (in Joules) absorbed per unit mass (kg) of the material when its temperature increases by 1 K (or 1 °C). Its units are J/kg/K or J/kg/°C.
Subtracting the formula
Let m be the mass of the system and c be the specific heat capacity of the system. Let ΔT be the change in temperature of the system.
Then the amount of heat supplied (Q) is the product of the mass m, specific heat capacity c and change in temperature ΔT and is given by,
Q = c × m × ΔT
Types of Heat Transfer
Heat transfer basically occurs in three ways.
- Conduction
- Convection
- Radiation
Conduction
Conduction is a type of heat transfer that occurs by transmitting heat from one side of a substance or object to the other. Heat transfer is always from high temperature to low temperature.
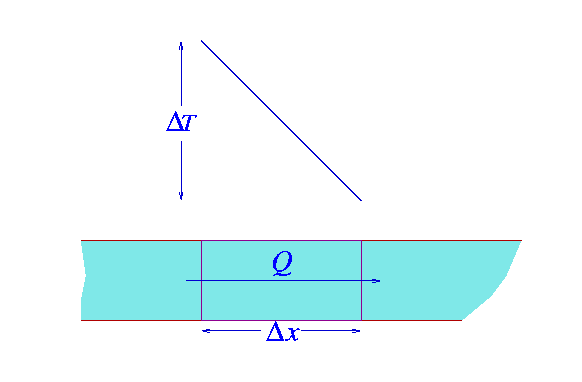
Q = kA(THot-TCold)t/d
Where,
Q: is heat transferred through conduction
k: is thermal conductivity of the material
A: is the area of the surface
THot is the temperature of the hot surface
TCold is the temperature of the cold surface
t: is time
d: is the thickness of the material
Convection
Convection is a type of heat transfer that occurs between a solid surface and a fluid. Heat is transferred through currents in the fluid. It may arise from temperature differences within the fluid or between the fluid and the boundary surface and the effect of this difference on density.
The transfer of heat through liquids and gases is called convection. The formula for heat transferred by the process of convection is expressed as:
Q = HcA(THot-TCold)
Q :is heat transferred through convection
Hc :is the heat transfer coefficient
A :is the area of the surface
THot :is the temperature of the hot system
TCold :is the temperature of the cold system
Radiation
Radiation or irradiation is the emission or transfer of energy in the form of electromagnetic waves or particles.The formula for heat transferred by the process of radiation is expressed as:
Q = σ (THot – TCold)4A
Q: is heat transferred through radiation
σ: is Stefan Boltzmann Constant
THot :is the temperature of the hot system
TCold :is the temperature of the cold system
A :is the area of the surface
Stefan Boltzmann Constant (σ) is calculated as:
σ = 2.π5 KB4 / 15 h3 c2 = 5.670367(13) × 10-8 J . m-2. S-1 . K-4
σ: is Stefan Boltzmann Constant
pi(π): ∼= 3.1415
kB :is Boltzmann constant
h :is Planck’s constant
c :is speed of light in vacuum
Sample Heat Transfer Problems
Problem 1: A system with a mass of 10 kg and an initial temperature of 200 K is heated to 450 K. Specific heat capacity of the system is 0.91 KJ/kg K. Calculate the heat gained by the system in this process.
Solution:
According to question,
Mass, m = 10 kg
Specific heat capacity, c = 0.91 KJ/kg K
Initial temperature, Ti = 200 K
Final temperature, Tf = 450 K
Change in temperature, ΔT = 450K – 200K = 250KUsing the heat transfer formula,
Q = c × m × ΔT
Q = 0.91 x 10 x 250
Q = 2275 KJTherefore the total heat gained by the system is 2275 KJ.
Problem 2: The specific heat of iron is 0.45 J/g°C. What mass of iron is required for a heat transfer of 1200 Joules if the temperature change is 40°C?
Solution:
According to question,
Specific heat of iron, c = 0.45 J/g°C
Change in temperature, ΔT = 40°C
Amount of heat transferred, Q = 1200 JUsing the heat transfer formula,
Q = c × m × ΔT
m = Q /(c x ΔT)
m = 1200 /(0.45 x 40)
m = 66.667 gTherefore required mass of iron for a heat transfer of 1200 Joules is 66.667 grams.
Problem 3: Consider two water columns at different temperatures separated by a glass wall of length 3m and width 1.5m and a thickness of 0.005m. One water column is at 380K and the other is at 120K. Calculate the amount of heat transferred if the thermal conductivity of glass is 1.4 W/mK.
Solution:
According to question,
Thermal Conductivity of glass, k = 1.4 W/mK.
Temperature of first water column, THot= 380K
Temperature of second water column, TCold = 120K
Area of the glass wall separating two columns, A = length x width = 3m x 1.5m = 4.5m2
Thickness of the glass, d = 0.005mUsing the heat transfer formula for conduction,
Q = kA(THot-TCold)t / d
Q = 1.4 x 4.5 (380-120) / 0.005
Q = 327600 WTherefore, amount of heat transferred is 327600 Watts.
Problem 4: Calculate heat transfer through convection if the heat transfer coefficient of a medium is 8 W/(m2 K) and the area is 25 m2 and the temperature difference is 20K.
Solution:
According to question,
Heat transfer coefficient, Hc = 8 W/(m2 K)
Area, A = 25m2
Change in temperature, (THot – TCold) = 20KUsing the heat transfer formula for convection,
Q = HcA(THot-TCold)
Q = 8 x 25 x 20
Q = 4000 WTherefore, amount of heat transferred through convection is 4000 Watts.
Problem 5: Calculate the heat transferred through radiation between two black bodies at temperatures 300K and 430K and the area of the medium is 48 m2. (Given Stefan Boltzmann Constant, σ = 5.67 x 10-8 W/(m2K4) ).
Solution:
According to question,
Temperature of hot body, THot= 430K
Temperature of cold body, TCold = 300K
Change in temperature, (THot – TCold) = 430K – 300K = 130K
Area, A = 48 m2
Stefan Boltzmann Constant, σ = 5.67 x 10-8 W/(m2K4)Using the heat transfer formula for radiation,
Q = σ (THot-TCold)4 A
Q = 5.67 x 10-8 x 1304 x 48
Q = 777.3 WTherefore, amount of heat transferred through radiation is 777.3 Watts.
Source: geeksforgeeks




