We continue the laws of thermodynamics with the second law of thermodynamics. In our previous articles, we talked about it in general and made detailed explanations about the zeroth law, the first law of thermodynamics and the second law of thermodynamics.
In this article, what is the third law of thermodynamics? We will try to find answers to our questions such as what are its limitations and what are its limitations?
What is Third Law of Thermodynamics?
The third law of thermodynamics is sometimes defined as ‘relating to the properties of systems in equilibrium at absolute zero temperature’ as:
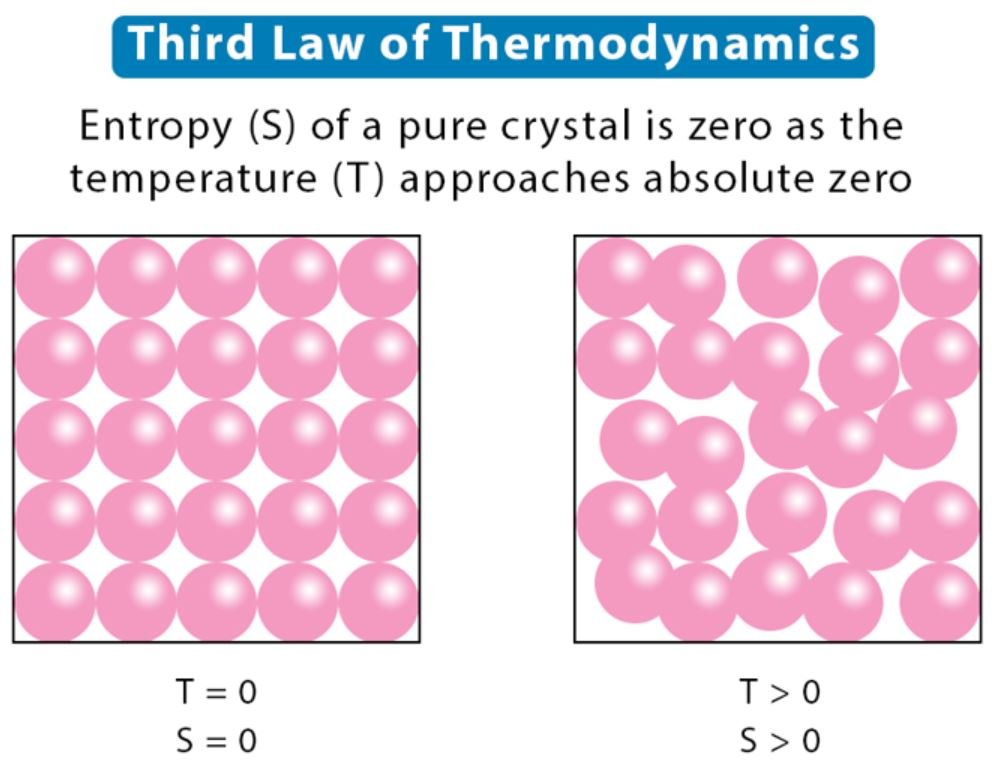
“The entropy of an ideal crystal is exactly zero at absolute zero kelvin.”
That is, at -273.15 degrees Celsius, the entropy of the ideal crystal is exactly equal to zero and the substance is stable and motionless at this temperature.
This explanation is also called the “Tofur law”. However, it is explained as the third law in high school, college and undergraduate curricula.
At zero kelvin, the system must be in a state where the minimum possible energy is present. And this statement of the third law is true if the ideal crystal has only one minimum energy state. Entropy is related to the number of possible microstates. And quantum mechanics; It shows that for systems containing a particular collection of particles, there is only one unique state (also called the ground state) with minimum energy.
If the system does not have a well-defined order (e.g. the order is glassy), then in practice some finite entropy will remain when the system is brought to very low temperatures. Because it becomes locked in a non-minimal energy configuration. The constant value is called the residual entropy of the system.
The Nernst-Simon statement of the third law of thermodynamics relates to thermodynamic processes and whether it is possible to achieve absolute zero in practice:
“As the temperature approaches 0 K, where dense systems refer to liquids and solids, the entropy change associated with any dense system undergoing a reversible isothermal process approaches zero.”
A simpler formulation of the Nernst-Simon expression could be:
“It is impossible for any process, no matter how idealized, to reduce the entropy of a system to absolute zero by limited operations.”
Physically, the Nernst-Simon expression; It implies that it is impossible for any procedure to bring a system to absolute zero temperature with a limited number of steps.
Formula of the Third Law of Thermodynamics
Consider a closed system in internal equilibrium. Since the system is in equilibrium, there is no irreversible process and therefore entropy production is zero. Temperature gradients occur in matter during heating, but if heat is introduced slowly, the associated entropy production can be kept low. The increase in entropy due to the added heat δQ is then given by the second part of the second law of Thermodynamics. This section expresses the entropy change of a system going through a reversible process as follows:
Consequences of the Third Law
Can absolute zero be achieved?
The third law is equivalent to the following statement: “It is impossible for any process, no matter how idealized, to reduce the entropy of a system to absolute zero by limited operations.”
According to the third law, why T=0 cannot be reached is explained as follows: Suppose that by changing the parameter X from X2 to X1, the heat of a substance can be reduced in a process with constant entropy.
A multistage nuclear demagnetization can be considered in which the magnetic field is turned on and off in a controlled manner. If there was an entropy change at absolute zero, T = 0 could be reached in a limited number of steps. However, there is no entropy change at T=0. So an infinite number of steps would be needed.
Source: Wikipedia