Thermodynamic cycles are cycles that involve a working fluid that undergoes one or more state changes (thermodynamic processes) and returns to its initial state by producing work or energy or transferring energy.
During a closed cycle, the system returns to its initial thermodynamic state of temperature and pressure. In thermodynamic systems, all cycle steps such as heat and work are independent of each other. The application of the first law of thermodynamics in systems returning to the initial state is as follows:
ΔE=Eout-Ein=0
It was stated above that there is no change in the energy of the system at the end of the cycle. Ein refers to the heat and work input in the system, Eout refers to the heat and work output in the system. The first law of thermodynamics also states that the net heat input in a cycle equals the net work output. (For heat, Qin is considered positive and Qout is considered negative.)
What is Thermodynamic Cycles
A thermodynamic cycle is a series of thermodynamic actions that bring the system to its initial state even if the process is performed repeatedly. Thermodynamic cycles are used to explain how engines that convert heat into work work.
The thermodynamic cycle is a closed cycle in which there are many changes due to temperature, pressure and volume, but the initial and final states are equal. Continuity is ensured with this cycle. For example, vehicle engines are generally 4-stroke and repeat constantly. Or it provides continuous cooling thanks to the refrigerator cooling cycle. Without thermodynamic cycles, the vehicle would not continue to run or the refrigerator would not cool.
There are many types of cycles in thermodynamics, and the important ones are listed below.
- Carnot Cycle
- Rankine Cycle
- Otto Cycle
- Diesel Cycle
- Brayton Cycle
- Stirling Cycle
Let’s briefly examine these cycles.
Carnot Cycle
The Carnot cycle is a special thermodynamic cycle introduced by Sadi Carnot in the 1820s and developed by Benoît Paul Émile Clapeyron in the 1830s and 1840s.
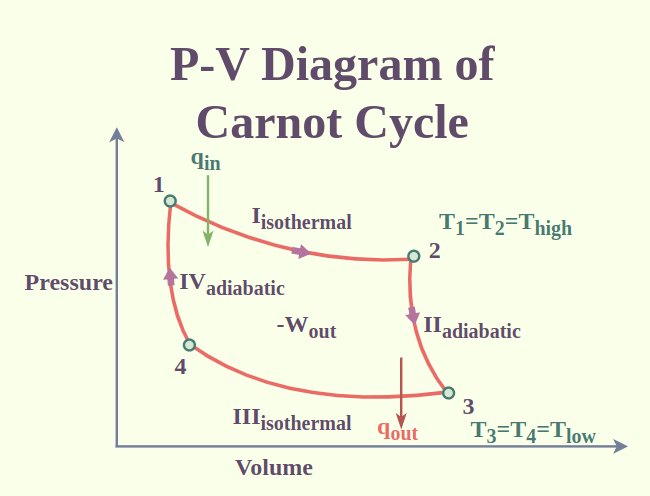
Converting heat to work, or converting work to heat, is the most efficient cycle that currently exists. The Carnot cycle consists of four reversible processes.
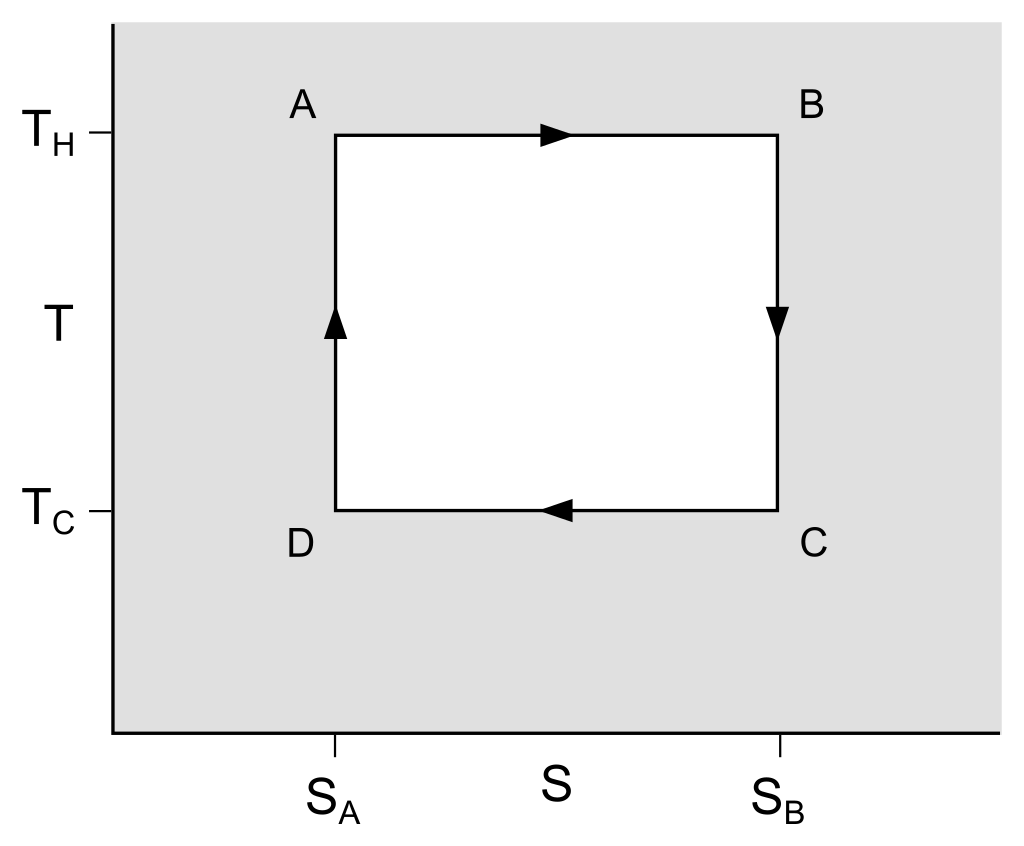
Isothermal expansion: During this step, the gas expanding (increasing in volume) causes the piston to do work. Gas expansion proceeds by the absorption of heat from high temperature. (between A-B)
Adiabatic expansion: In this step, the piston and cylinder are considered to be thermally insulated, so there is no heat loss. The gas continues to expand and do work. The gas cools to TC temperature due to expansion. (between B-C)
Isothermal compression: At this moment, the gas gives work to its surroundings, causing heat release towards low temperature. (between C-D)
Adiabatic compression: Again, the piston and cylinder are considered thermally insulated. The work done in this step causes the gas to be compressed and its temperature to rise to TH temperature. At this point, the gas has returned to its initial state in the first step. (between D-A)
The Carnot cycle can be considered as a closed loop in the temperature-entropy graph.
η = 1−TH/TC
TC : is the temperature of the cool supply, and
TH : is the temperature of the hot repository.
Rankine Cycle
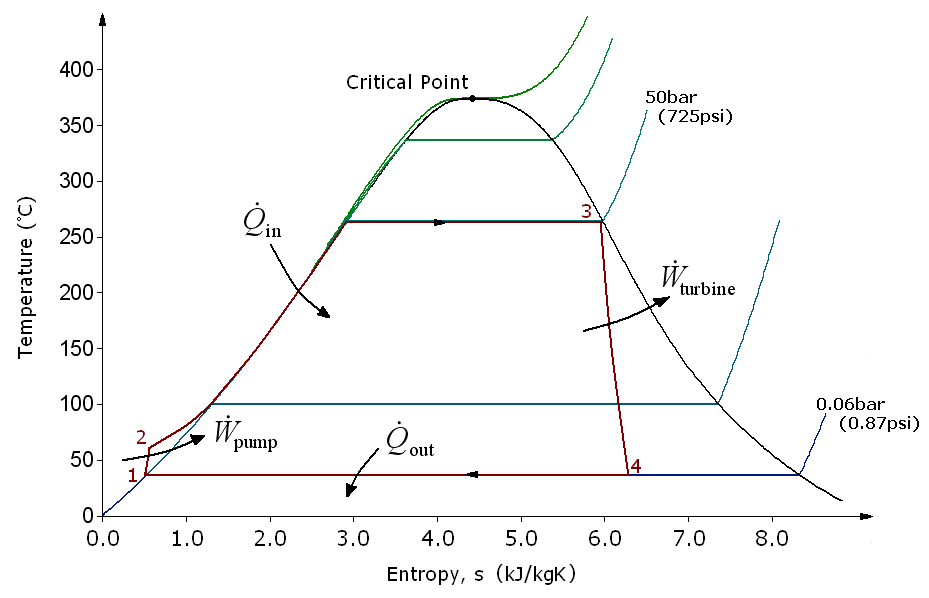
The Rankine cycle is the ideal cycle for power plants using steam. Turning the water into superheated steam in this cycle and turning it into saturated liquid again in the condenser eliminates many difficulties encountered in practice in the Carnot cycle.

There are four processes in the Rankine cycle. The states are identified by numbers (in brown) in the T–s diagram.
| Name | Summary | Explanation |
|---|---|---|
| Process 1–2 | Isentropic compression | The working fluid is pumped from low to high pressure. As the fluid is a liquid at this stage, the pump requires little input energy. |
| Process 2–3 | Constant pressure heat addition in boiler | The high-pressure liquid enters a boiler, where it is heated at constant pressure by an external heat source to become a dry saturated vapour. The input energy required can be easily calculated graphically, using an enthalpy–entropy chart (h–s chart, or Mollier diagram), or numerically, using steam tables or software. |
| Process 3–4 | Isentropic expansion | The dry saturated vapour expands through a turbine, generating power. This decreases the temperature and pressure of the vapour, and some condensation may occur. The output in this process can be easily calculated using the chart or tables noted above. |
| Process 4–1 | Constant pressure heat rejection in condenser | The wet vapour then enters a condenser, where it is condensed at a constant pressure to become a saturated liquid. |
Otto Cycle
An Otto cycle is an idealized thermodynamic cycle that describes the functioning of a typical spark ignition piston engine. It is the thermodynamic cycle most commonly found in automobile engines.