We will try to explain the first law of thermodynamics to you. In our previous articles, we discussed the Laws of Thermodynamics in general and explained the zeroth law of Thermodynamics in detail.
So what is the first law of Thermodynamics? What does it explain? Let’s look at it together.
What is First Law of Thermodynamics?
The first law of thermodynamics is also known as “conservation of energy”. Energy cannot be created from nothing; Existing energy cannot be destroyed; it just transforms from one shape to another. For any cycle of a system, heat exchange and work exchange during the cycle must be equal to each other in the same unit system and proportional to each other in different unit systems.
The accuracy of these statements has been observed through experiments, but they cannot be proven.
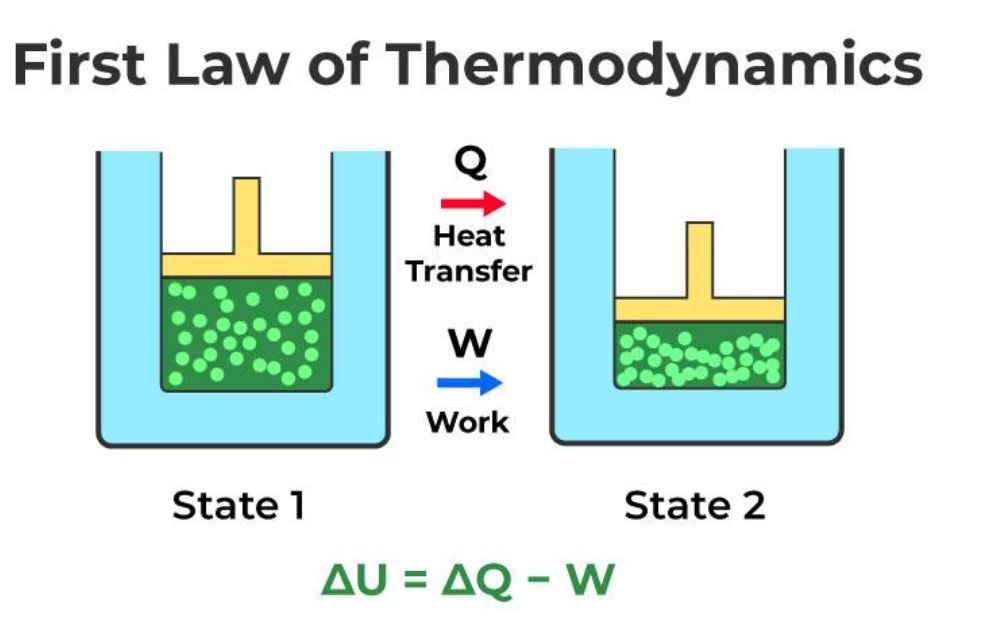
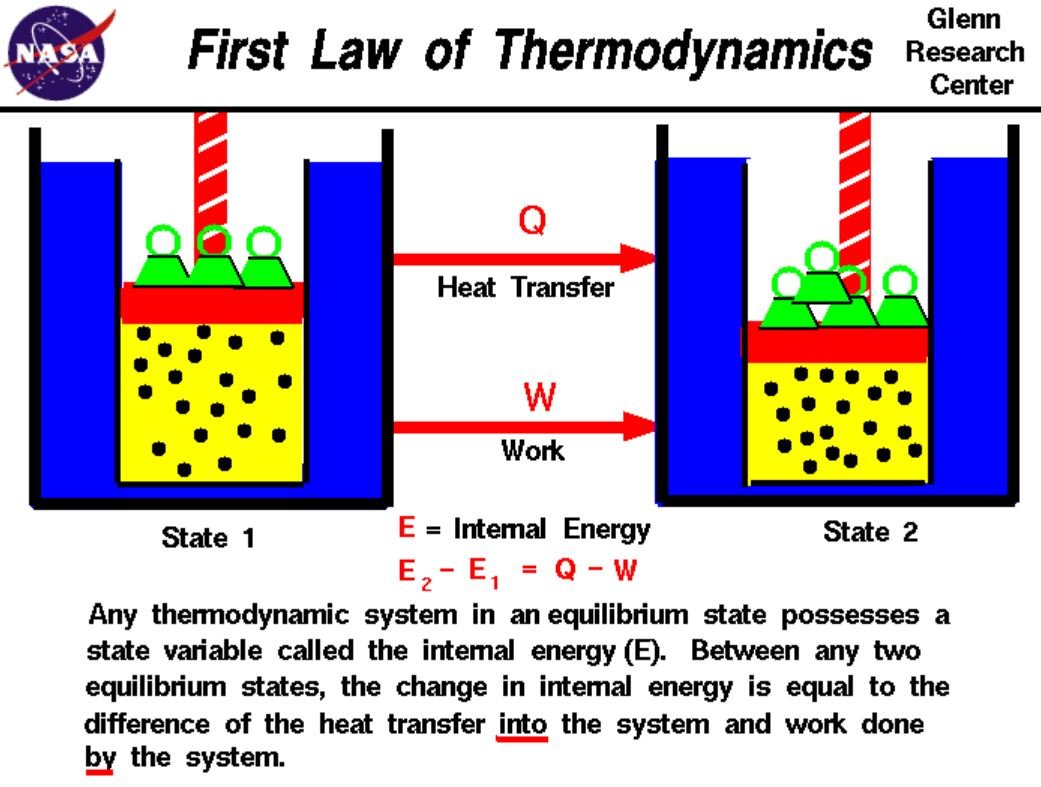
The change in internal energy of a system is the sum of the heat given to the system and the work applied to the system by the environment.
U2 – U1 = Q + W
Internal energy is a state variable at equilibrium in a thermodynamic system. The difference in internal energy between two systems is equal to the heat transferred to the system minus the work done by the system.
According to the First Law of Thermodynamics, the energy of the universe is constant and does not change. Energy can be transferred between the system and the environment, but it cannot be produced or destroyed. The law is primarily concerned with energy states that arise as a result of work and heat conduction.
First Law of Thermodynamics Formula
According to the first law, some of the heat given to the system is used to change internal energy, while the rest is used by the system to do work. The mathematical expression of the first law of thermodynamics is given as follows:
ΔQ = ΔU + ΔW
Where
ΔU is the change in internal energy of the system,
ΔW is the work done by the system,
ΔQ is the heat supplied to the system.
The product of the applied pressure and the change in volume as a result of the applied pressure is the work done for a closed system:
W = – P ΔV
Where
- P denotes the system’s constant external pressure, and
- V denotes the volume change.
This is referred to as Pressure-Volume work.
The internal energy of a system rises or falls in response to work interactions that occur across its limits. When work is done on the system, the internal energy increases, but it decreases when work is done by the system. Any heat exchange between the system and its surroundings alters the system’s internal energy. However, the total change in internal energy is always zero since energy remains constant (according to the first rule of thermodynamics). If the system loses energy, it is absorbed by the surroundings. If energy is absorbed into a system, the energy must have been released by the environment:
ΔUsystem = −ΔUsurroundings
Where
- ΔUsystem is the change in the total internal energy of the system, and
- ΔUsurroundings is the change in the total energy of the surrounding.
Limitations of First Law of Thermodynamics
- The first law of thermodynamics has a limitation because it does not specify anything about the direction of heat flow.
- It is not possible to reverse the procedure. In reality, heat is not completely converted into labor. If it were possible to convert all heat into work, we could move ships across the ocean by extracting heat from ocean water.
- It makes no distinction between whether the process is spontaneous or not.