Although fuel cells have been in use for a very long time, they have become widespread recently. The fuel cell was first discovered in 1838, but it did not find much use. It came to the fore again in 1959, when NASA used fuel cells in its space program, and its development continued.
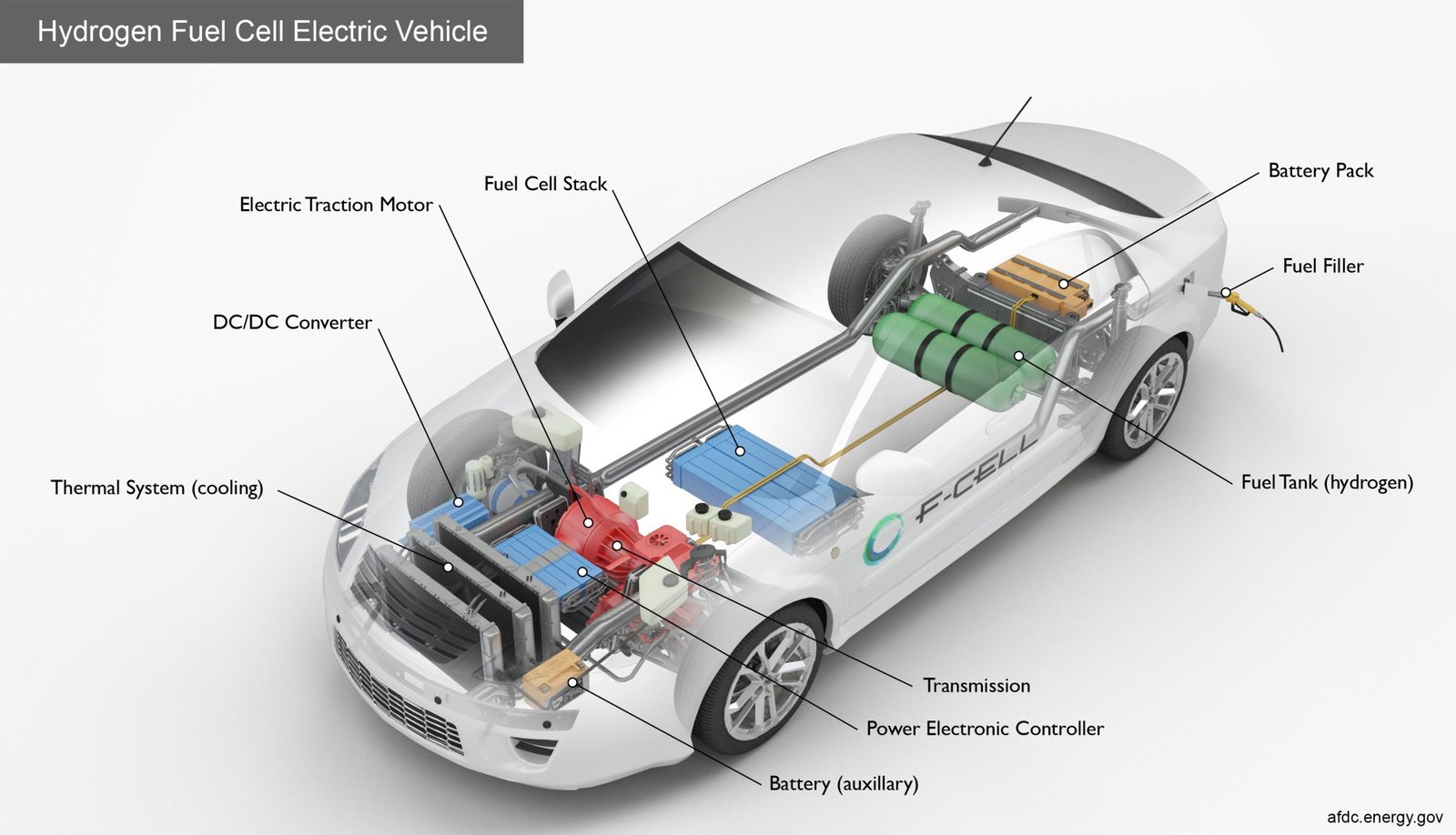
Nowadays, many uses of fuel cells have emerged. Finally, vehicles started to be produced with fuel cells. Prototype vehicles hit the market.
What is Fuel Cells
A fuel cell is an electrochemical cell that produces electricity from fuel. Although many different fuels are used, today the focus is on hydrogen.
In order for electricity production to be continuous, it is important that the fuel source is not interrupted. For this, a constant fuel source and an oxidizing element (usually oxygen) are required.
These fuel cells continue to produce power until the fuel and oxygen used are exhausted.
Fuel cells directly convert the chemical energy of the fuel into electrical energy. Fuels such as hydrogen (H2), carbon dioxide (CO2), methane (CH4), propane (C3H8), methanol (CH3OH) and others were used to produce electrical energy in the cells shown below. While there are many types of fuel cells on the market, the most popular is the hydrogen-oxygen fuel cell.
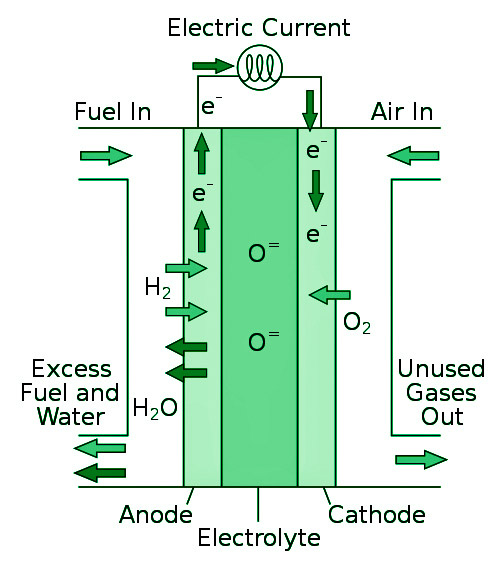
Types of Fuel Cells
We mentioned that there are many types of fuel cells. The 10 most common fuel cells are listed below.
- Hydrogen-Oxygen fuel cell
- Polymer Electrolyte Membrane (PEM) Fuel Cell
- Phosphoric Acid Fuel Cell
- Solid Acid Fuel Cell
- Alkaline Fuel Cell
- Molten Carbonate Fuel Cell
- Microbial fuel cells (MFCs)
- Solid Oxide Fuel Cells (SOFCs)
- Zinc-Air Fuel Cell (ZAFC)
- Direct Methanol Fuel Cell (DMFC)
Hydrogen-Oxygen Fuel Cell
Bacon first invented the Hydrogen-Oxygen fuel cell in 1959. For this reason, it is also called Bacon cell. It became very popular after it was used by NASA in the Apollo space program.
Two porous carbon electrodes are impregnated with a suitable catalyst such as platinum (Pt), silver (Ag), cobalt oxide (CoO), and so on in a basic H2–O2 fuel cell. The electrolyte is a concentrated solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH) that fills the gap between two electrodes. A porous carbon electrode bubbles hydrogen gas (H2) and oxygen gas (O2) into the electrolyte.
- At the anode, 2H2 (g)+4OH– (aq) → 4H2O (l)+4e–
- At the cathode, O2 (g)+2H2O (l)+4e– → 4OH– (aq)
- Overall reaction, 2H2 (g) + O2 (g) → 2H2O (l)
The Polymer Electrolyte Membrane (PEM) Fuel Cell
- Proton exchange membrane fuel cells are another name for these cells (or PEMFCs).
- These cells function at temperatures ranging from 50 degrees Celsius to 100 degree Celsius.
- The electrolyte used in PEMFCs is a polymer that can conduct protons.
- A PEM fuel cell is made up of bipolar plates, a catalyst, electrodes, and a polymer membrane.
- Despite its environmentally benign applications in transportation, PEMFCs can also be utilised for fixed and portable power generation.
Phosphoric Acid Fuel Cell
- Phosphoric acid is used as an electrolyte in these fuel cells to channel the H+.
- These cells operate at temperatures ranging from 150-200 Celsius.
- Because phosphoric acid is non-conductive, electrons must go to the cathode via an external connection.
- Because the electrolyte is acidic, the components of these cells corrode or oxidise with time.
Solid Acid Fuel Cell
- The electrolyte in these fuel cells is a solid acid substance.
- At low temperatures, the molecular structures of these solid acids are organised.
- At higher temperatures, a phase shift can occur, resulting in a significant increase in conductivity.
- CsHSO4 and CsH2PO4 are two examples of solid acids (cesium hydrogen sulphate and cesium dihydrogen phosphate respectively).
Alkaline Fuel Cell
- This was the fuel cell that served as the major source of power for the Apollo space programme.
- An aqueous alkaline solution is employed in these cells to saturate a porous matrix, which is then used to separate the electrodes.
- These cells’ operating temperatures are relatively low.
- These cells are quite effective. Along with power, they generate heat and water.
Molten Carbonate Fuel Cell
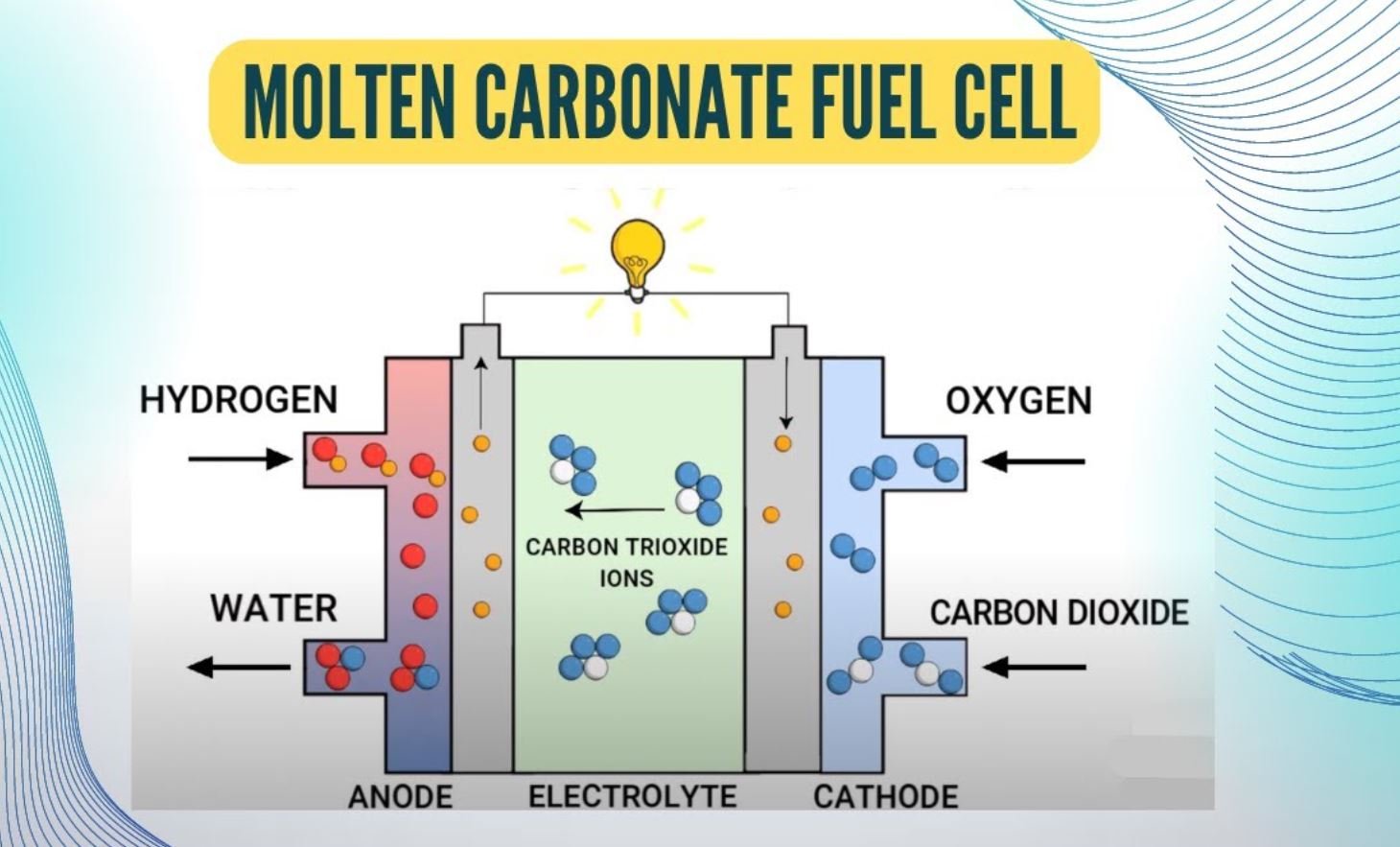
- Lithium potassium carbonate salt is employed as the electrolyte in these cells. At high temperatures, this salt becomes liquid, allowing carbonate ions to migrate.
- These fuel cells, like SOFCs, have a relatively high working temperature of 650 Celsius.
- Because of the high working temperature and the presence of the carbonate electrolyte, the anode and cathode of this cell are prone to corrosion.
- These cells can run on carbon-based fuels like natural gas and biogas.
Solid Oxide Fuel Cells (SOFCs)
Solid oxide fuel cells employ a hard, non-porous ceramic substance as the electrolyte and operate at temperatures between 500 and 1000 degrees Celsius. A solid oxide electrolyte is used in SOFCs to transport negative oxygen ions from the cathode to the anode. SOFCs have an efficiency of 50–60 percent.
- At the anode: 1/2O2+2e–→O
- At the cathode: H2+1/2O→H2O+2e–
- The overall cell reaction: H2+12O2→H2O
Satellites and space capsules employ SOFCs to generate electricity. It is mostly employed in big, high-power applications such as industrial generating plants.
Zinc-Air Fuel Cell (ZAFC)
The Zinc-Air Fuel Cell (ZAFC) is a kind of fuel cell that was created in the United States for use in vehicles. The electrolyte is an aqueous alkali solution such as potassium hydroxide, and the electrode reactions are as follows:
- Anode: Zn+2OH–→Zn(OH)2+2e–
- Cathode: O2+2H2O+4e–→4OH–
- Overall Reaction: 2Zn+O2+2H2O→4Zn(OH)2
It is used as an alternative fuel for vehicles.
Direct Methanol Fuel Cell (DMFC)
Methanol is utilised as a fuel in this subclass of proton-exchange fuel cells. The key benefit of this fuel cell is the ease with which stable liquid fuel methanol may be transported. Polymer membrane serves as the electrolyte, and the electrode reactions are as follows:
- Anode: CH3OH+H2O→6H++CO2+6e–
- Cathode: 3/2O2+6H++6e–→3H2O
- Net reaction: CH3OH+3/2O2→CO2+2H2O




